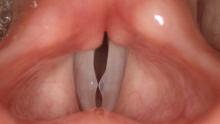
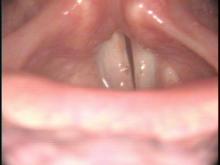
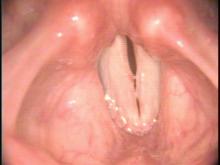
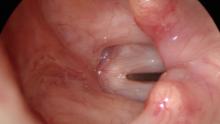
High Definition Laryngology
Abstract
A voice laboratory is an expensive proposition when the most current equipment options are purchased; consequently, a voice examination can be an expensive proposition as well. An examiner having the latest equipment and high definition technology offers the ability to see larynx anatomy and function in finer detail than in the past. However, even if one does not have the highest technology available, knowing what can be seen with the best equipment helps to understand and interpret the findings when using lower technology.
Low technology, high definition techniques allow the examiner to improve upon high quality equipment or to make up for the lack of high quality equipment. An optimum combination of equipment and technique leads to a cost-effective, high yield and accurate diagnosis for laryngeal problems. It also leads to an improved understanding of how the larynx actually functions to produce clear sound both for speaking and for singing. This is what I call high definition laryngology.
If you are a physician, this may help you decide what equipment to utilize or purchase. It will provide you with ideas for new techniques to image the vocal cords and their function more precisely. If you a patient, you will understand better how to assess the report and recommendations received from your laryngologist.
Goals
- To understand the most important component of laryngology is the video recording of the image. The capacity to review an image several times reveals many additional details.
- To understand how the most commonly used endoscope, the very cost-effective flexible fiber-optic endoscope, artificially alters our perception of the larynx. If the examiner keeps this visual alteration in mind, a better diagnosis can be made, even with these slightly blurred and honey-combed images.
- To understand how variations in lighting alter our perceptions of what we see and specifically how SD (standard definition) rigid and flexible endoscopes can offer complementary views of the larynx, the combination resulting in essentially a high definition perception of the larynx.
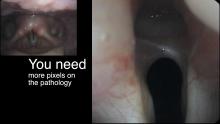
- To understand how topical anesthesia - at very little cost and only a small addition of time - can turn an SD image into essentially an HD image by navigating the endoscope closer. Closeness is equivalent to more pixels of the pathology and consequently by definition is a higher resolution image. Consequently individuals who can only afford the minimal amount of equipment can learn to obtain essentially HD images of pathology with low-budget equipment.
- To understand the capabilities of the highest resolution images that can be obtained with high definition cameras from Toshiba, Olympus and Pentax for both rigid and digital chip-on-tip endoscopes. Whether or not you can afford an HD endoscope, you should understand what you might be missing.
- To understand how the added visual qualities of false color imaging such as NBI by Olympus or iScan by Pentax highlight vasculature. Neo-vascularization readily identifies the borders of many lesions, some malignant and some benign.
Case study
A 72-year-old female woke up hoarse after a mitral valve surgery. Six months after her problem began, she came into my office still with a hoarse voice and difficulty breathing.
She says, “I had my heart valve replaced and became very ill. My daughter says that I had a tube in my throat to breath for me for 10 days. The first I can recall, I had a very weak voice. My doctor said just to wait, it almost always gets better with time.
About six weeks after surgery, I developed difficulty breathing and went to the emergency room. My voice was getting stronger at that time. They ran quite a few tests and told me that I did not have anything serious, at least I did not have a blood clot in my lungs. They sent me to a lung doctor.”
They actually treated with inhaled racemic epinephrine and bronchodilators. She received a Chest X-ray, CT Scan of the lungs, and a VQ (ventilation-perfustion) Scan. They noted in the record that she did not have a pulmonary embolism and felt that she probably had emphysema. She was referred to a pulmonologist.
She continued, “The lung doctor examined my lungs with a camera. He told me that I had a vocal cord problem and referred me to an ENT doctor. She put a scope in and told me that my vocal cords were paralyzed. She sent me to a specialist in vocal cords. He told me that both vocal cords were paralyzed and that I needed a test called an EMG and he put me on a pill to stop stomach acid.”
He placed her on pantoprazole twice a day and referred her to a neurolaryngologist.
She continues after being prompted, “The next specialist told me that the EMG was abnormal and he recommended injecting Botox into my vocal cord last week. I let him do that and my breathing has gotten worse and I make more noise now with breathing. Even just getting up from a chair to walk makes me short of breath and causes a lot of noise.”
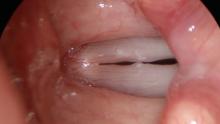
All of these visits have taken about four months after she first became short of breath. She makes obvious noise when she breathes inward and breathing appears effortful. When I place an endoscope in her pharynx, her vocal cords are resting near the midline. When she breathes in, she makes a high pitched noise and the right vocal cord draws inward and vibrates. When she breathes out, the vocal cords shorten in length and the mid-membranous portion of the right vocal cord moves laterally. The upper portion of the arytenoids hide the posterior, cartilaginous portion of the vocal cords, which do not appear to move laterally very far during inspiration.
Since I cannot see all of the vocal cords nor can I see beneath her vocal cords, I ask her permission and topically anesthetize her vocal cords with lidocaine.
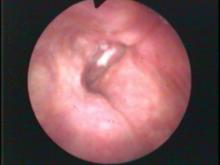
The significant findings are a band of scar tissue running underneath the vocal processes, fixing the vocal processes about 2 mm apart at the anterior cartilage tip. The right vocal cord, which had been injected with OnabotulinumtoxinA about one week earlier, was atrophic. With any increase in her rate of inspiration, the right membranous vocal cord would draw medially and flutter against the left vocal cord. During phonation the upper portion of the arytenoids would move closer together and touch, while the left vocal process appeared to move about 1 mm closer to the right vocal process. The rest of her trachea, carina and left and right mainstem bronchi were normal.
Whenever there is an intubation, there are two significant risks to be considered in the differential diagnosis. Is this a neurologic injury or is this a traumatic mucosal injury? An endotracheal tube cuff which is inflated too tightly and ends up located in the immediate subglottic area can put pressure on the anterior branches of the recurrent laryngeal nerves as they pass beneath the thyroid cartilage and partially or completely paralyze one or both of them, limiting abduction and adduction of the vocal cords. It will also typically lead to atrophy of the injured thyroarytenoid muscle(s).
After even as little as a two hour intubation, pressure ulcers will frequently develop on or near the vocal processes. With longer intubations, larger ulcerations and fibrinogen may create a bridge between the ulcers, which ultimately epithelializes and contracts, pulling the vocal processes towards each other.
Either condition, scar contracture or the synkinetic reinnervation that occurs after nerve injury, ends up bringing the vocal cords closer together in the midline. In my experience, scar contracture ends up restoring the voice and limiting breathing sooner (often by 2 months) than neurologic synkinetic reinnervation (often by four months). Consequently, both diagnoses need to be considered whenever there is a limitation of vocal cord motion after an intubation.
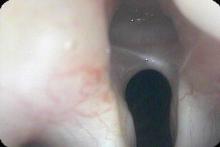
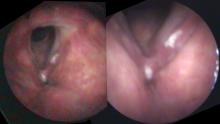
In our example case, when the larynx is visualized with the endoscope at a level near the tip of the epiglottis, only the lack of abduction is perceived. After we topically anesthetized her vocal cords and passed the endoscope beneath the arytenoids, we viewed the movement of the vocal processes with inspiration, expiration and attempted phonation. We viewed the mass of both vocal cords, effectively assessing the thyroarytenoid muscles. We viewed the posterior subglottis where scar tissue typically forms. We passed the endoscope into the trachea where there can be a secondary injury from the endotracheal tube cuff to the walls of the trachea and creating a second stenosis. By moving the standard definition endoscope immediately adjacent to the posterior glottis, we effectively filled about 200 pixels of the endoscopic’s 640 by 480 pixels with the scar tissue creating her glottic stenosis.
If we compare this close view, where a 200 x 200 pixel are of the image is filled with the pathology to a typical endoscopic overview image of the entire larynx where the pathology is effectively hidden by the upper portion of the arytenoids, we can appreciate that moving in the endoscope close to the pathology becomes a high definition view of the pathology where we initially had a 0 x 0 pixel view or one could say, a zero-definition view of the pathology when viewed from the level of the epiglottis.
The harm of using up 6 months of someone’s life before obtaining an accurate diagnosis, the financial expense of all the non-essential tests performed on her, the reliance on a difficult to interpret test - a laryngeal EMG - for clinical decision making, the harm done when the unilateral injection of botulinum toxin led to the right thyroarytenoid muscle no longer remaining tense during inspiration and Bernoulli effect inducing further functional obstruction of the airway which increases with air hunger, the unnecessary proton-pump inhibitor... all of this makes me feel the additional 15 minutes required to topically anesthetize larynx and pass the endoscope through the vocal cords to the carina at the initial visit is an incredibly valuable, low technology, high definition laryngology maneuver. This is putting the pixels on the pathology.
Low technology, high definition laryngology is using inexpensive techniques to put pathology onto more pixels of the endoscope, no matter what endoscope the examiner already has. Low definition laryngology is where examiners may have high technology equipment, even a high technology education but;
- they don’t realize where to aim the endoscope, or
- don’t take the time to record quality images of the pathology or
- do not understand the need for using low technology wisely to bring the pathology into focus and/or
- do not review for mistakes to learn from them.
Voice Laboratory
A voice laboratory might contain some or all of the following:
- Endoscopes (rigid and/or flexible)
- Camera & Processor (integrated or external)
- Stroboscope
- High Speed
- Hardware and software for recording video with audio
Rigid endoscopes
Rigid endoscopes tend to come in two configurations. Based on the angle of view, they are termed 70° and 90° endoscopes. Sometimes a quick focus ring is added, although the claim is made that this absorbs some light. In general, the glass transmitting the image in a rigid endoscope, carries more light than flexible endoscopes.
The benefits of the rigid endoscope are that it provides a high clarity image. It can be attached to a standard definition camera and may be upgraded with high-definition cameras. It is particularly valuable for viewing mucosal edge lesions of the vocal cords. When attached to a stroboscope mucosal lesions on the margin of the membranous vocal cord stand out in high contrast against the background of the dark trachea.
The potential pitfalls of the rigid endoscope are that it usually provides only a single perspective view, various anatomic structures can obstruct the image (uvula, base of tongue, epiglottis, posterior pharyngeal masses, supraglottic squeeze), gagging may preclude an examination altogether and it has a relatively shallow depth of field.
Flexible endoscopes
There are a number of variations in the equipment available for flexible laryngoscopy. The main differentiation is between fiber-optic technology and chip technology.
Fiberoptic endoscopy
Typically the flexible fiber-optic endoscope is attached to a separate camera. The flexible aspect of this technology allows the endoscope to be passed through the nose, typically reducing the gag reflex during the examination. It allows the endoscope to easily be passed closer to the vocal cords and even beyond the structures of the larynx. Most laryngeal function is retained during a flexible examination. The angle of view of the structures of the larynx can be modified to provide various perspectives on the same pathology.
Flexible endoscopes have a wider angle lens than rigid endoscopes and a wider angle perspective than the human eye. Consequently close objects appear relatively larger than objects farther away. There is a high depth of field so more of the image is in focus at a given time. Endoscopes with a more gentle curve in the tip and the curve close to the tip of the endoscope are easy to maneuver. One significant appeal for this equipment is that fiber-optic technology is relatively inexpensive by medical standards.
On the downside, although the camera can be updated from standard definition to high definition, the images provided by this technology are inherently quality limited by the flexible glass fibers carrying both light to the interior of the larynx as well as transmitting the image of the larynx back to the camera to which it is attached. These relatively large glass fibers are visible in any image produced. When the image is recorded, the pixelation of the glass fibers interact with the pixels on the recording device and create a moire effect. Attempts to diminish the honeycomb image and moire effect produced by this technology involve a loss of resolution by blurring the image either physically or electronically.
A major downside to fiber-optics is that glass fibers absorb light (and when they break over time, the absorption becomes complete and leads to a black spot in the image). There is ultimately a limit to how much light can be passed through the glass fibers and auto gain features of the cameras can lead to artifact. Digital noise produced by increasing video gain alters both the color and clarity of the images.
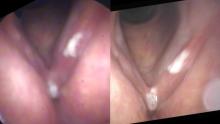
Same person, same day, same camera. The image on the left is taken from farther away than the image on the right. As the camera moves closer, more light is reflected and the auto gain feature reduces the digital noise.
Digital noise is particularly evident when the endoscope is relatively far away from the pathology. For example, with the endoscope at the level of the tip of the epiglottis, there is significant loss of light at the level of the vocal cords several centimeters away. Since most users have the auto-gain function turned on, electronic amplification of the image occurs without the examiner even sensing the degradation of the image. As electronic gain increases, more pixels in the image are assigned colors that don’t actually exist. The image becomes more pixilated. Additionally, as capillaries become blurred, the image becomes more apparently red.
Chip endoscopy
Chip on tip technology derived it’s name from miniaturizing the electronic sensor or chip and then moving it from the camera (which was typically attached to the endoscope eyepiece) onto the tip of the endoscope, transmitting images electronically to an external processor. Since the image no longer passes through the light fibers, the double pixelation issue is removed and the images are much clearer. As chips become smaller, higher resolution chips lead to higher definition images and/or smaller endoscopes. Additional processing of the image such as selective light absorption can alter the images before they are recorded.
Camera & Processor
What is meant by “definition?”
Although any combination of x by y dimensions could be utilized the video industry has settled on a relatively few standardized formats. The most popular, based originally on TV formatting, has become labeled standard definition. When televisions were still analog, the standard called NTSC consisted of 525 lines, the aspect ratio was 4:3 and the images were recored at 30 frames per second. Each frame consisted of two fields which were interlaced so that 60 fields were recorded per second. As digital technology took over this standard, the 4:3 aspect ratio turned into 640 x 480 pixels utilized to record the same image (some of the 525 vertical lines were used for other things during the analog era). Many other compromises have been made over time for technical reasons as well as commercial reasons and Wikipedia as a nice overview if you desire further information. Technology has not remained still and more and more pixels are able to be processed and stored. Various compromises have led to high definition video recording settling on a 16:9 aspect ratio Some of the shorthanded utilized to express video recording is listed below.
Video format classification
- SD
- 525i - 640 horizontal by 480 vertical pixels
- HD
- 720p - 1280 x 720 pixels
- 1080i - 1920 x 1080 pixels
Graphic format classification
- Highly variable, almost any x:y variation of pixels
Determining the video output of the processor affects the resolution that should be captured. Capturing at a higher resolution than the camera outputs results in wasted hard disk storage. Capturing at a lower resolution than the camera outputs results in blurry videos.
Light
The camera and processor are usually sold as a package. Often the light source is integrated into the processor. However, for laryngology, separating the light cable from the camera allows for connection to a stroboscope. Xenon, halogen and LED lights are being used for laryngeal endoscopy. Each colors the image in a slightly different way. Even the camera used for recording and the screen used for viewing the video recording alters the perceived color of the laryngeal tissues.
I have detailed my thoughts on two major producers of laryngeal endoscopes, Olympus and Pentax. Even when the resolution of the cameras are identical, videos of an individual larynx appear different depending on the camera used to obtain the images.
Transmission
Carrying audio and video signals along a cable requires a format. Some of the terms that you might run into include:
- DVI - Digital Video interface (carries analog or digital signals, SD or HD, but no audio)
- HDMI - High Definition Multimedia Interface (SD, HD, video, audio)
- SDI - Serial Digital Interface (single cable)
- HD-SDI - High Definition Serial Digital Interface
- Firewire 400, 800 -
- Thunderbolt (Apple)
Conversion
Basically, what comes out of your camera may not be what your computer’s hard disk wants to suck in. So unless your entire system is designed by one manufacturer, you may need to do a conversion between the camera and your computer. Also, since video consists of so much data, most conversion processes require hardware in order to do a conversion quickly enough not to lose frames on the video. So quite typically, the cable comes out of the camera processor carrying video to hardware converter which may combine the audio with video, convert the video to a different format and then plug into a computer.
Capture
I have been capturing video on Apple laptop computers for some time. There are various software programs that can grab live video. The one I currently use is designed to go with the hardware conversion box which encodes the video for Apple’s QuickTime video format. QuickTime seems to have remained a stable standard “wrapper” over the past 15 years. The codecs within QuickTime have changed over time but I can still view all of the video I have recorded with this program.
After capture, I organize and review the video in Apple’s Final Cut Pro video program. Another common program is Adobe’s Premiere video editing program.
Storage
No one wants to store every pixel that comes out of your endoscope. Consequently there are various compression and storage formats for video these are called codecs. Some of the more common ones are listed.
Video codecs & wrappers
Compression algorithms
- MPEG-4
- MPEG-2
- H-.264
- WMV (Windows Media Video)
- Apple ProRes 422
- DV
- quicktime (.mov)
I end up with a mix of formats on my hard drive. My old files are formatted in DV. I record my high-definition camera’s Using Apple ProRes 422. I store my standard definition videos in H.264. The hospital operating room cameras and endoscopes variously capture video in MPEG-4 and WMV formats.
Speaking of hard drives, my laptop rapidly fills up with high-definition video files. I currently copy them to a Pegasus RAID hard drive which makes two copies of each video so that when one hard drive dies, the video still exists elsewhere on the RAID drives. Every few weeks, I also store another copy of all the video files on a separate hard drive which I store at a different physical location. Redundancy is a key word for data files.
Slow Motion
There are two main ways to capture images of moving vocal cords: each way has trade-offs. Since video is typically captured at about 30 images per second and almost all vocal cord vibratory movement occurs at more than about 80 cycles per second, almost all images of vibrating vocal cords will appear blurred on standard video recording. After one has seen enough slow-motion video recordings, one can often infer from these blurred images what is actually happening to the vocal cords in real time.
A significant improvement on standard video occurs with a stroboscope. A sensor detects the pitch of a voice and triggers a stroboscope to flash light at one or two beats more per second than the vibration of the vocal cords. The video then records slow apparent motion of the vocal cords. The trade-off with stroboscopy is that when there is irregular vibration, the strobe light may flash irregularly in this artifact may create a false impression of the actual physical movement of the vocal cords.
High-speed video records several thousand frames per second, thus recording actual vocal court motion. Some of the trade-offs with this technique are that it requires a large amount of storage, it can be difficult to capture actual pathologic movement of the vocal cords since you have to record in short bursts and it can take a long time to review this video to find the pathologic motion. As a newer technology, it is also relatively more expensive than stroboscopy.
Selective Color imaging
Since video images consist of red, green and blue channels, it is possible to selectively filter out some of the light on the individual color channels. Both Olympus and Pentax have made use of this and electronically filter certain bands of light in the blue and green wavelengths which are absorbed by hemoglobin. Blood, normally viewed as read in the images, then appears dark or black in the video recordings adding additional contrast with the surrounding tissue.
This makes the capillary vasculature of the larynx pop out in the images. While the capillaries are visible using the normal lighting algorithms, emphasizing capillaries tends to lead the eye to various laryngeal conditions. In particular, hemorrhage and dilated capillaries occur with trauma and infections, but most notably capillaries are dilated and generate specific patterns as tumors grow and require or induce an increased vascular supply for their metabolic needs. Additionally, very thin films on the vocal cords can be more readily visually identified because the films obscure the expected normal capillary architecture beneath them.
Recording & Reviewing Video
Ultimately, many things happen in the larynx very quickly and sometimes pathology may be highly visible recognizable on as little as only a single frame of a video recording. This means that during live recording, the problem might be present but not be visually identified. Even during the quick overview of the recording that I perform while the patient is in the examination room and during my explanation of the problem to the patient, I slip through the video to the most pertinent pictures in order to describe the problem. On a regular basis, I review the video again as I am developing a report on a patient. I try to find optimal individual frames of the video that demonstrate the pathology. During this detailed review, as I sometimes play the video frame by frame, I often notice additional detail.
Sometimes after surgery or some type of intervention or even just the passage of time, when something doesn’t turn out as expected, I return to prior videos and review them in light of the new video information and I will find discover details that I missed on prior reviews. Consequently, I find that the recording of a video is valuable not only for finding the immediate problem, but is equally valuable for teaching myself about the significance of audio and visual findings that I might have initially missed.
Low Technology
Low technology is really the most valuable part of a High Definition endoscopic examination. The high definition image obtained does not depend on money. It depends on the examiner’s knowledge, skill and patience. In our example patient at the beginning of this article, no matter what definition the endoscope record, the pathology occupied zero pixels of the endoscope when it was placed at the level of the epiglottis, a common location for many endoscopic laryngeal recordings. By applying topical anesthesia and maneuvering the endoscope beneath the upper portion of the arytenoids, the pathology could be seen just inferior to the vocal processes. By the time the tip of the scope was close to the vocal processes, I estimate that about 400 square pixels of Low Technology, High Definition imaging was infinite, merely by supplementing a typical laryngeal examination with an additional 10 minutes and some topical lidocaine.
Here are some selected ways that low technology can be utilized to obtain a high definition laryngeal exam.
Closeness
Occasionally the endoscope can be maneuvered close to the larynx just because a patient can tolerate it, or because a sensory nerve injury allows the endoscope to actually touch the larynx without triggering a gag or initiating a laryngospasm. Sometimes it is possible to maneuver the endoscope in between the vocal cords during inspiration and back it up during expiration as the vocal cords tend to close.
More typically though, topical anesthesia is the laryngologist’s best friend. With the addition of 4 to 5 mL of topical 4% lidocaine, typically dripped on the vocal cords in 1 mL aliquots over several minutes, the larynx can be completely anesthetized and an endoscope can usually be maneuvered anywhere in the larynx or trachea. The first aliquot of anesthetic makes the patient cough and distributes medication around the entire larynx, pharynx and subglottis. I ask the patient to phonate while I add additional topical lidocaine and this generates a laryngeal gargle. After the fourth milliliter is dripped and gargled and I wait for an additional 2 to 3 minutes, the topical anesthesia is complete. (For more information on local anesthesia in laryngology: Thomas, JP: “Topical Anesthesia for Office Based Laryngeal Interventions” in Principles and Practice of Lasers in Otorhinolaryngology and Head and Neck. (Ed. Oswal, V; Remacle, M; Jovanvic, S; Zeitels, SM; Krespi, JP; Hopper, C) Kugler Publications 2014, ISBN 978-90-62992-32-4)
Camera orientation
While the image captured by endoscopes is typically round, there is enough transmission of light with rigid endoscopes too mechanically zoom some cameras without abundant loss of light and resolution. When combined with the newer high definition ratio of 16:9, vibrating vocal cord anatomy nicely aligns with this horizontal visual field in terms of length: width ratio, utilizing more of the pixels available on the camera. Compared to my typical placement of the camera on a flexible fiber-optic endoscope or the orientation of the camera chip on integrated chip endoscopes, this rotation of the external camera attached to the rigid endoscope 90°, aligns the vibrating edges of the vocal cords horizontally on the stretched high definition video monitor.
Laryngeal Maneuvers
Choose a vowel
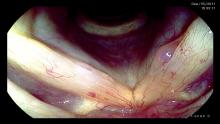
Since most consonants are produced at the level of the palate, tongue and lips, it is not necessary to visualize connected speech during a laryngeal examination. Vowels are produced in the pharynx. The vocal cords are primarily responsible for the production of sound. They primarily modulate frequency and volume. Consequently If we eliminate words during our examination we reduce inadvertent movement of the flexible endoscope by palatal and tongue movement. We want to keep the pharynx as open as possible and the epiglottis as far forward as possible. The vowel /i/ provides for this maximal pharyngeal opening.
Ahhh sound in left photo. Eeeeee or /i/ sound in right photo. Same person, same day, same camera.
Alter Pitch
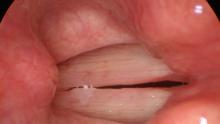
We can record our examination at both low and high pitch. In fact, if we identify a particular pitch during the vocal capabilities pattern matching portion of our examination that triggers dysphonia, we should make an attempt to record that pitch during our endoscopic examination. Apart from finding a specific pitch that triggers dysphonia, high pitch activates the cricothyroid muscle to lengthen the vocal cord and the thyroarytenoid muscle to intrinsically tension the vocal cord. As the vocal cords lengthen, any lesion along the vibratory margin will tend to be pushed out into the field of view, particularly from the rigid endoscopes vertical viewpoint. At high pitch, the vocal cords are also reaching their maximum tension which will aggravate any pre-existing stiffness and make the vibratory impairment more visible.
Low pitch phonation on left. High-pitched phonation on right. The vocal cord calluses on the margin are more visible at high pitch
Recording at low pitch removes any inadvertent or obligate compensation by the cricothyroid muscle. Activation of the cricothyroid muscle may occur in nonorganic voice disorders. It almost always occurs in neurologic disorders involving paresis of either the thyroarytenoid muscle or the lateral cricoarytenoid muscle. As the patient reduces this intrinsic compensation by the cricothyroid muscle, a weakened thyroarytenoid muscle will allow the vocal cord to flutter and oscillate more laterally. With the removal of intrinsic compensation by the cricothyroid muscle, a weakened lateral cricoarytenoid muscle will allow the vocal process to cant laterally creating an obtuse angle between the vocal process and the membranous vocal cord.
In the left photo, the vocal cords are viewed at high pitch and the cricothyroid muscle tightens the paretic and weak right vocal cord. In the right photo, there is no compensation and the weak, atrophic right vocal cord flutters far laterally during oscillation. The thinness of the right vocal cord is also apparent.
Another inadvertent compensation is that typically patients surprisingly do not want to sound abnormal during an examination, even though that the raison d’être for their office visit. This is particularly true for singers. They often convince themselves that abnormal vocal sounds are the results of poor technique. In order to avoid audible impairments in their upper vocal range they increase their subglottic pressure and consequently their volume. Small gaps, small elevations and minor stiffness are overcome with increased subglottic pressure. By eliciting from the patient low-volume sound production, smaller vocal impairments can be visually discovered during the examination.
Higher volumes can augment neurologic and muscular weakness visual findings by causing the weakened vocal cord to vibrate abnormally. A non-tense thyroarytenoid muscle will flutter, break up into two or more oscillatory segments and oscillate lateral to its axis with an infinite open phase.
Alter Pitch
High pitch
- Highlights vocal margin
- Augments stiffness
Low pitch
- Highlights weakness
- Typically removes compensation
- From superior laryngeal nerve (cricothyroid muscle)
Alter Volume
Low volume
- Highlights gaps
- Highlights stiffness
- Highlights elevations
High volume
- Highlights weakness
Monitoring the vocal cords closely during quiet respiration can detail subtle neurologic findings. Fasciculations are often noticed during quiet breathing when the larynx is relatively still. These can be seen on the arytenoid relatively easily. They are also visualized within the laryngeal ventricle on the superior surface of the vocal cord in a denervated thyroarytenoid muscle.
The timing of abduction and adduction are also well assessed during quiet respiration. During expiration, partial adduction typically occurs and during inspiration, partial abduction occurs. The degree of abduction and adduction should be symmetric. With acute denervation the degree of motion is less on the injured side. Later, as varying degrees of synkinetic reinnervation occur, the timing of motion can become asymmetric. Additionally, with synkinetic reinnervation, the resting position of the vocal process on the inside becomes more medial.
Height differences can really only be appreciated well when the flexible endoscope is placed between the arytenoids, nearly parallel to the axis of vocal cords. From the same posterior view, oriented along the axis of the vocal folds, the mass of each thyroarytenoid muscle can be compared. Sniffing augments abduction, lengthening the vocal cords while they are pulled further apart than during quiet inspiration and an atrophic vocal cord will thin even further.
Summary
When you have the money; spend it for the new high technology. When you don’t; use what you have, better.
James P. Thomas, MD